In-person is the ideal scenario for assessing abnormal movements; however, screening for, diagnosing, and assessing TD can all be readily done virtually
Ways to Assess TD
Expert analysis of video recordings of the Abnormal Involuntary Movement Scale (AIMS) has been used as the primary means of measurement in clinical trials
Virtual visit
The need to assess TD is not diminished in the virtual setting
- Assessing AIMS items 1, 2, 3, 4, 5, and 7 can be readily done during a video call when the patient is seated
- With additional camera positioning, examination of all 7 AIMS items, including item 6, is possible
Benefits of virtual visits include:
- You can observe the patient in their own environment
- You can bring the family members into the conversation
- Patients may be less likely to miss appointments
FACIAL & ORAL MOVEMENTS
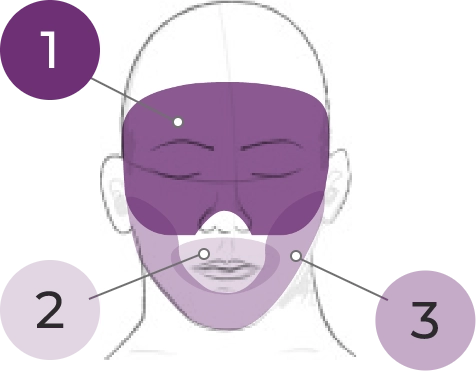
1. Muscles of facial expression
2. Lips and perioral area
3. Jaw
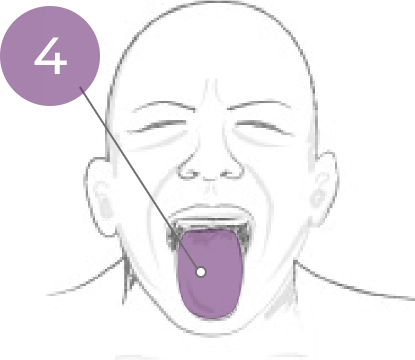
4. Tongue
EXTREMITY MOVEMENTS
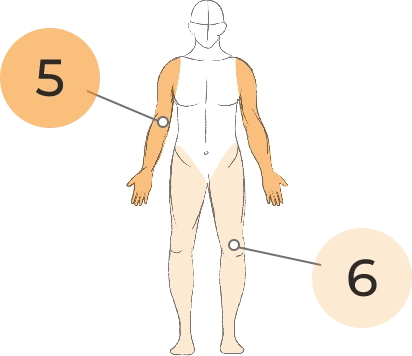
5. Upper (arms, wrists, hands, fingers)
6. Lower (legs, knees, ankles, toes)
TRUNK
MOVEMENTS
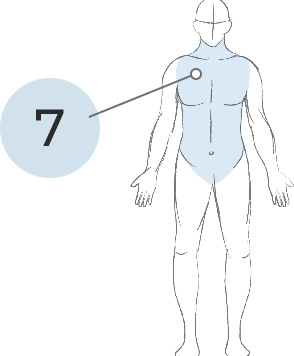
7. Neck, shoulders, hips
Telephone calls are not ideal; however, assessment for abnormal movements still needs to be done
- Focus on asking patients whether they or others around them notice any abnormal movements; if there are movements, ask about impact
- Start the conversation and, if you suspect TD, follow up in person or via video call
- Consider asking the patient to have a caregiver record a video, which can be sent for you to review
PerfecTD:

Question 1 of
True or False: A patient’s movements can only be assessed during an in-person visit.
Answer: False
You can assess a patient’s movements on a virtual visit, and you can probe about movements on a telephone call.
True or False: Assessing 6 of 7 AIMS items can be readily done during a video call.
Answer: True
Assessing 6 of 7 AIMS items can be readily done during a video call when the patient is seated. With additional camera positioning, examination of all 7 AIMS items is possible.
Thank you for completing
Screening and Assessment: Chapter 4
Virtual Screening and Assessment of TD
Summary:
Video assessments can be used effectively to screen for and diagnose TD.
Hear From the Expert
Visit the overview page to access a video of
Dr Rakesh Jain discussing the critical importance of screening for and assessing TD.
Chapter 4 References:
American Psychological Association. https://www.apa.org/practice/guidelines/telepsychology.
Caroff SN et al. J Clin Psychiatry. 2020;81(2):19c12983.
Citrome L. Psychiatry & Behavioral Health Learning Network. https://www.hmpgloballearningnetwork.com/site/pcn/multimedia/treating-td-covid-19-era-5-steps-success.
Dorfman S, Henault F. CMI/Compas. 2020.
Dorsey R et al. International Parkinson and Movement Disorder Society. https://www.movementdisorders.org/MDS/Scientific-Issues-Committee-Blog/cwmandtpdbt.htm.
Fernandez HH et al. Neurology. 2017;88(21):2003-2010.
Gulko C. Medpage Today. https://www.medpagetoday.com/resource-centers/tardive-dyskinesia-contemporary-approaches/tardive-dyskinesia-tips-conducting-patient-focused-exams/3347.
Guy W. ECDEU Assessment Manual for Psychopharmacology: Revised. Rockville, MD; 1976:534-537.
Jain R. Psych Congress Network. https://www.hmpgloballearningnetwork.com/site/pcn/article/can-aims-exam-be-conducted-telepsychiatry.
Kopelovich SL et al. Community Ment Health J. 2021;57(3):405-415.
McEvoy JP. Clinical Care Options Neurology/Psychiatry. https://www.clinicaloptions.com/neurology-psychiatry/programs/2021/tardive-
dyskinesia/clinicalthought/ct1/page-1.
Srinivasan R. Tremor Other Hyperkinet Mov (N Y). 2020;10.
STABLE National Coordinating Council Resource Toolkit Workgroup. https://provider.medmutual.com/pdf/STABLE_toolkit.pdf.